Context
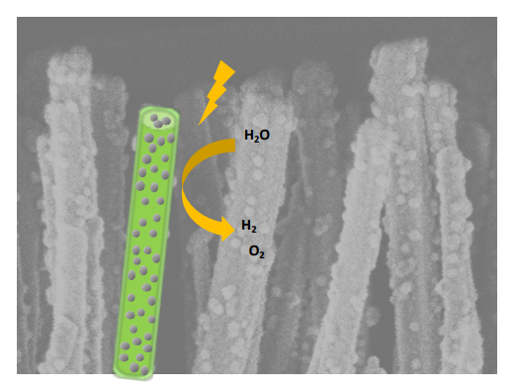
The rapid development of photoelectrochemical (PEC) processes is driven by the need for clean energy (H₂ production, CO₂ photoreduction) and pollution remediation.1 These processes rely on semiconductor (SC) structures that convert solar photons into charge carriers to drive redox reactions. An ideal material must efficiently absorb sunlight, separate and transport charge carriers, and catalyze reactions while remaining stable in water.2 However, no single material meets all these criteria, making heterostructures, such as SCs combined with co-catalyst nanoparticles, a promising alternative. Despite advances, achieving both high photoconversion and long-term stability remains a challenge. While photovoltaic-derived SCs offer high efficiency but low stability, oxides are more stable yet suffer from poor visible light absorption and charge mobility.3 Cost-effective photoelectrodes are crucial for large-scale PEC adoption, particularly for decentralized H₂ production in sun-rich regions.
Zinc oxide (ZnO) has gained attention as a photoanode for PEC water oxidation due to its high electron mobility, optical properties, abundance, and low toxicity.4 However, its large bandgap (3.2 eV) limits absorption to UV light, and it undergoes photocorrosion under UV exposure, leading to decomposition in extreme pH conditions. Consequently, ZnO is only stable within a narrow pH range (7–9), restricting its practical use in PEC applications.
The objective
This PhD project aims atdevelopingnew photoelectrodes associating ZnOnanorods and cocatalyst nanoparticles inn order to allow a better management of the photogenerated charge carriers. A fundamental challenge in this field is to unravel the mechanisms governing photogenerated charge transport, particularly at the semiconductor/cocatalyst/electrolyte interface. A precise understanding of charge carrier dynamics— mobility, separation, and recombination—is essential for optimizing photoelectrode performance. However, this requires well-defined hierarchical nanostructures, which remain difficult to obtain and control, posing a major obstacle to advancing our knowledge in this area.
By combining our expertise in material design and the access to states of the art high resolution spectroscopy techniques, this project aims to gain deeper insights into charge carrier mobility and recombination to achieve highly efficient and stable photoelectrodes.
The work will involve:
- Synthesizing nanostructured composites based on well-aligned ZnO nanorod arrays grown on a support via wet chemistry. These ZnO nanorods will then be functionalized with nanoparticles using a self-assembly strategy involving colloidal suspensions.5 The green chemistry approach, respectful of the environment (aqueous medium, low temperature, quantitative yields, etc.) will be chosen.
- Investigating the photoelectrochemical properties of the synthesized nanostructures with spectral resolution.6 By fine-tuning key parameters—such as the chemical composition and size of nanoparticles, as well as the length, cross-section, and density of nanorods—the study will establish structure-property relationships to identify the most efficient and stable photoelectrode.
- Conducting a rational analysis of the photoelectrochemical mechanism.The new Attolight® platform will enable the acquisition of cutting-edge data, offering unprecedented insights into charge carrier mobility and recombination at the ZnO/nanoparticle interface.7 This deeper understanding will drive the design of optimized nanostructured photoelectrodes, enhancing light-to-chemical energy conversion efficiency.
The labs
This project will be carried out as part of the joint initiative between the Interdisciplinary Thematic Institutes for Hierarchical & Functional Materials for Health, Environment, and Energy (HiFunMat) and New Insights into Materials and Light (Mat-Light 4.0). The PhD candidate will work at the intersection of three laboratories, all of which have longstanding collaborations in the design of advanced nanomaterials with a strong emphasis on sustainability, including green chemistry, energy conversion and storage, and pollutant remediation.
- The Institute of Physics and Chemistry of Materials of Strasbourg (IPCMS) will provide state-of-the-art facilities for the synthesis of nanostructured composites, along with advanced characterization techniques such as TEM, SEM, XRD, FTIR, and granulometry.
- The Institute of Chemistry and Processes for Energy, Environment, and Health (ICPEES) will offer access to spectral-resolved photoelectrochemical analyses, including linear sweep and cyclic voltammetry under chopped simulated solar light, quantum yield measurements, and electrochemical impedance spectroscopy.
- The Institute of Materials Science of Mulhouse (IS2M) hosts the SEM-Attolight® platform, enabling time-resolved studies of charge carrier mobility and recombination mechanisms via cathodoluminescence, photoluminescence, Raman spectroscopy, and conductivity measurements using four nano robots. All of these techniques will be performed as a function of temperature(10 to 300 K).
This thesis will be attached to the Physics and Physical Chemistry doctoral school (ED 182). The PhD student will be supervised by Benoit P. Pichon and will be mainly based in Strasbourg at IPCMS. He / She will closely interact with Thomas Cottineau (ICPEES) and Jean Luc Bubendorf (IS2M). The measurements carried out on the Attolight® platform will be scheduled for campaigns lasting several days, with accommodation in Mulhouse provided by IS2M.
IPCMS and ICPEES are both located on the CNRS campus in Strasbourg, just a 20-minute bike ride from the city center. IS2M is in Mulhouse, approximately 1 hour and 30 minutes from Strasbourg by train, with swift and frequent connections.
The phD student will be employed by CNRS for 36 months (start on October 1st) and will be member of IPCMS, ICPEES and IS2M laboratories.
A permanent office space will be provided at IPCMS and ICPEES.
Eligibility criteria
We are looking for an enthusiastic, rigorous and self-motivated candidate with good communication skills to join our research team. The successfull candidate should:
- Have a Master’s degree (or comparable) in material chemistry, physical chemistry, chemical engineering or related disciplines from a recognized university.
- Have Strong interest in experimental physical chemistry/chemical physics/materials science.
- Be able to work independently as much as in a multi-disciplinary international team.
- Have a good command of English
Please submit the following application materials to Prof. B. Pichon benoit.pichon[at]unistra.fr
- A Cover letter describing background and interest in the proposed project (max. twopages);
- A Curriculum Vitae (max. three-pages; summarizing education, positions, pedagogical experience, administrative experience and other qualifying activity);
- A letter of recommendation and two additional reference names
- (If applicable) proof of English language competence.
- Copies of educational certificates, both bachelor and masters (academic transcripts only)
Application details
Provisional start date : 01/10/2025
Provisional defence date : 30/09/2028
References
(1) Kim, J. H.; Hansora, D.; Sharma, P.; Jang, J.-W.; Lee, J. S. Toward Practical Solar Hydrogen Production – an Artificial Photosynthetic Leaf-to-Farm Challenge. Chem. Soc. Rev.2019, 48 (7), 1908–1971. doi.org/10.1039/C8CS00699G. (2) Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater.2016, 1
(2), 15010. doi.org/10.1038/natrevmats.2015.10. (3) Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; Narushima, R.; Okunaka, S.; Shibata, N.; Takata, T.; Hisatomi, T.; Domen, K. Photocatalytic Solar Hydrogen Production from Water on a 100-M2 Scale. Nature2021, 598 (7880), 304–307. doi.org/10.1038/s41586-021-03907-3. (4) Liu, C.-F.; Lu, Y.-J.; Hu, C.-C. Effects of Anions and pH on the Stability of ZnO Nanorods for Photoelectrochemical Water Splitting. ACS Omega2018, 3
(3), 3429–3439. https://doi.org/10.1021/acsomega.8b00214.
(4) Liu, C.-F.; Lu, Y.-J.; Hu, C.-C. Effects of Anions and pH on the Stability of ZnO Nanorods for Photoelectrochemical Water Splitting. ACS Omega2018, 3 (3), 3429–3439. doi.org/10.1021/acsomega.8b00214.
Our references
(5) Azeredo, B.; Carton, A.; Leuvrey, C.; Kiefer, C.; Ihawakrim, D.; Zafairatos, S.; Gallart, M.; Gilliot, P.; Pichon, B. P. Synergistic Photo Optical and Magnetic Properties of a Hybrid Nanocomposite Consisting of a Zinc Oxide Nanorod Array Decorated with Iron Oxide Nanoparticles. J. Mater. Chem. C Mater. Opt. Electron. Devices2018, 6 (Copyright (C) 2019 American Chemical Society (ACS). All Rights Reserved.), 10502–10512. doi.org/10.1039/c8tc02680g.
(6) Favet, T.; Sharna, S.; Keller, V.; El Khakani, M. A.; Cottineau, T. (M,N) Codoping (M = Nb or Ta) and CoO Nanoparticle Decoration of TiO2 Nanotubes: Synergistic Enhancement of Visible Photoelectrochemical Water Splitting. Mater. Today Energy2023, 37, 101376. doi.org/10.1016/j.mtener.2023.101376.
(7) Bubendorff, J. L.; Ebothé, J.; El Hichou, A.; Dounia, R.; Addou, M. Luminescent Spectroscopy and Imaging of Textured Sprayed Er-Doped ZnO Films in the near Ultraviolet and Visible Regions. J. Appl. Phys.2006, 100 (1), 014505. doi.org/10.1063/1.2211347.